Composition of Protein
Proteins essentially contain carbon, hydrogen oxygen and nitrogen, a few have sulphur, phosphorus, iron and iodine. Actual composition of a protein depends upon its source. However, approximate value of their constituents may be given as under.
- Carbon 46-50%
- Oxygen 20-24%
- Hydrogen 6-7%
- Nitrogen 16-18%
- Sulphur 0.2-2%
- Phosphorus 0.1-1%
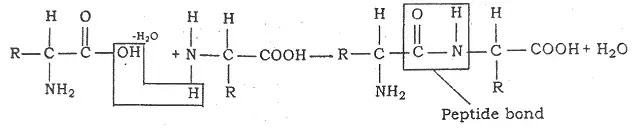
Proteins are formed by the combination of amino acids linked together by peptide bonds. During combination, last hydroxyl (OH) group of carboxyl of one amino acid combines with a (H) atom of another amino acid and forms a molecule of water.
The water molecule is removed and a bond between two amino acids is formed. This bond is known as peptide bond.
- When only two amino acids are linked together, it is called dipeptide if three tripeptide and when a large number of amino acids are links are called a polypeptide.
- Peptides form more complex molecules called peptones which form proteases and these in turn constitute the proteins.
Thus the protein is a long polypeptide chain containing a number of amino acids linked together.
- Insulin is considered to be the smallest protein which has fifty one amino acids. It is composed of two chains. The A chain consists of amino acids whereas B chain is composed of 30 amino acids. Both chains are joined together by s-s bonds.
On the basis of manner of arrangement of amino acids in polypeptide chain, protein structure is categorized into following four types -
(i) Primary structure
Amino acids remain linked by polypeptide bonds in a linear sequence one after another. e.g. myoglobin
(ii) Secondary structure
When a long polypeptide chain forms a spiral just like spring around an imaginary axis it is known as secondary structure. Stability is provided by the formation of hydrogen bonds between two amino acids at the point of turnings.
(iii) Tertiary structure
When the polypeptide chains are too long they exist as tightly coiled molecules i.e. the protein chains remain super folded.
This super folding of protein chains are known as tertiary structure. This super folding is maintained by the non-covalent interactions occurring between the side chains i.e. H-bonds, ionic and hydrophobic bonds.





No comments:
Post a Comment